Study Results:
Select Safety Information
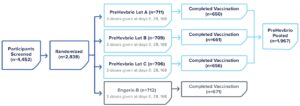
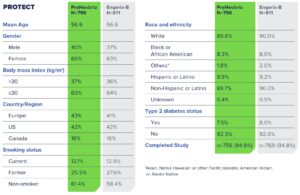
Safety Was Evaluated in Two Phase 3 Studies
Solicited Local and Systemic Adverse Reactions (Age 18-44 Years)1
Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination
a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization.
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization.
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization.
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis.
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Solicited Local and Systemic Adverse Reactions (Age 45-64)1
Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination
a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization.
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization.
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization.
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis.
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Solicited Local and Systemic Adverse Reactions (Age 65+ Years)1
Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination
a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization.
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization.
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization.
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis.
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
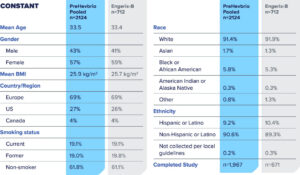
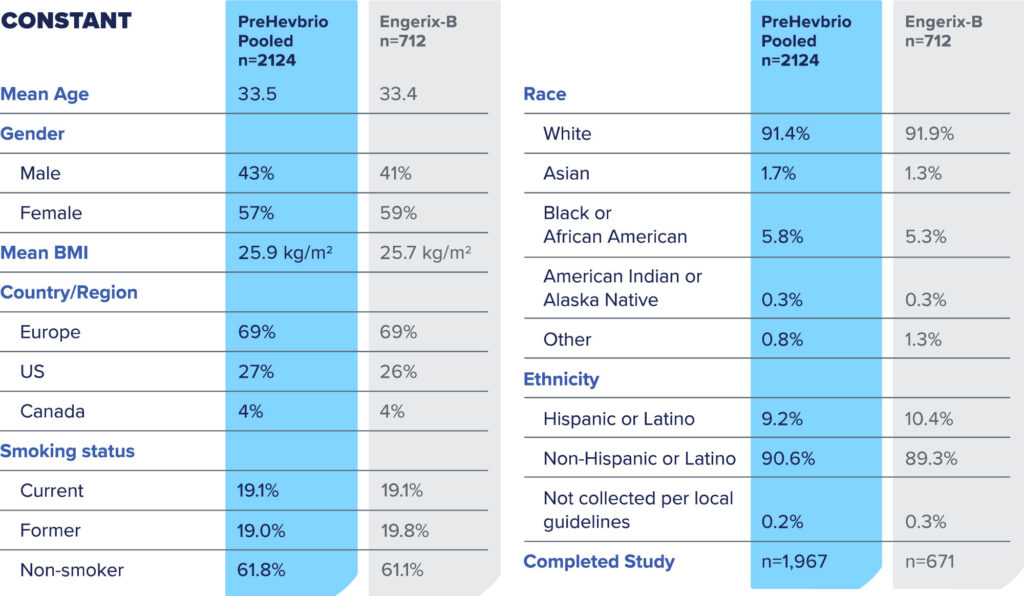
CONSTANT Phase 3 Study
Solicited Local and Systemic Adverse Reactions (Age 18-45 Years)1
Percent of Subjects Who Reported Local or Systemic Reactions Within 7 Days of Vaccination
a Grade 3 or greater pain and headache: defined as use of narcotic pain reliever or prevents daily activity; or ER visit or hospitalization.
b Grade 3 or greater tenderness: defined as significant discomfort at rest; or ER visit or hospitalization.
c Grade 3 or greater itching, fatigue and myalgia: defined as prevents daily activity; or ER visit or hospitalization.
d Grade 3 or greater redness: defined as > 10 cm or skin necrosis or exfoliative dermatitis.
e Grade 3 or greater swelling: defined as > 10 cm or prevents daily activity; or skin necrosis.
f Grade 3 or greater diarrhea and nausea/vomiting: defined as prevents daily activity or requires outpatient IV hydration; or ER visit or hospitalization.
Unsolicited Adverse Events (AEs)1
- In both studies, unsolicited adverse events, including serious and non-serious events, that occurred within 28 days following each vaccination were recorded on a diary card by all subjects.
- In both studies combined, unsolicited AEs that occurred within 28 days of any vaccination were reported by 48.3% and 48.4% of subjects who received PREHEVBRIO or Engerix-B, respectively. Unsolicited AEs in subjects who received PREHEVBRIO for which available information suggests a causal relationship to vaccination include injection site bruising (1.4%), dizziness/vertigo (1.1%), general pruritus/itchiness (0.2%), arthralgia (0.2%), urticaria/hives (0.2%) and lymphadenopathy/lymph node pain (0.1%).
Serious Adverse Events1
- In both studies, SAEs were collected from first vaccination through 6 months following the last vaccination. In both studies combined, SAEs were reported by 0.9% and 0.6% within 28 days of vaccination with PREHEVBRIO or Engerix-B, respectively. SAEs were reported by 2.5% of subjects in the PREHEVBRIO group and 1.6% in the Engerix-B group from the first vaccination through 6 months following the third vaccination. There were no notable patterns or numerical imbalances between vaccination groups for specific categories of serious adverse events that would suggest a causal relationship to PREHEVBRIO.
Indication & Important Safety Info
INDICATION
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
INDICATION
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PreHevbrio.
Immunocompromised persons, including those on immunosuppressant therapy, may have a diminished immune response to PreHevbrio.
PreHevbrio may not prevent hepatitis B infection, which has a long incubation period, in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common side effects (> 10%) in adults age 18-44, adults age 45-64, and adults age 65+ were pain and tenderness at the injection site, myalgia, fatigue, and headache.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women who received PreHevbrio during pregnancy. Women who receive PreHevbrio during pregnancy are encouraged to contact medinfo@vbivaccines.com or call 1-888-421-8808 (toll-free).
To report SUSPECTED ADVERSE REACTIONS, contact VBI Vaccines at 1-888-421-8808 (toll-free) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov
Please see Full Prescribing Information.
References
-
PreHevbrio Prescribing Information. Cambridge, MA. VBI Inc.