Study Results:
Immunogenicity
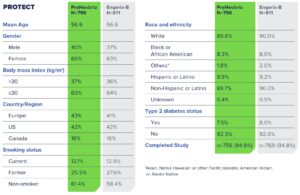
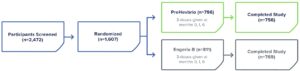
PROTECT—Phase 3 Clinical Trial to Evaluate Immunogenicity and Safety1
PreHevbrio was evaluated in a double-blind, randomized, two-arm, safety and immunogenicity study of adults (n=1,607), including those with well-controlled chronic conditions across 28 sites in Europe, the U.S., and Canada.1
More Adults Achieved Seroprotection with PreHevbrio in PROTECT1
PreHevbrio elicited non-inferior seroprotection rates (SPR) in adults age 18+, and statistically significantly higher SPR in adults age 45+, compared to Engerix-B
Seroprotection Rates at Day 196
* Seroprotection was defined as anti-HBs titres ≥10 mlU/mL; SPR was defined as the percentage of participants attaining seroprotection.
Seroprotection rates across age groups1
Seroprotection Rates at Day 196
Concentration of Anti-HBs antibody titers across age groups4
Antibody Titers at Day 196
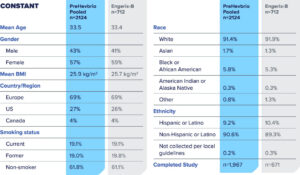
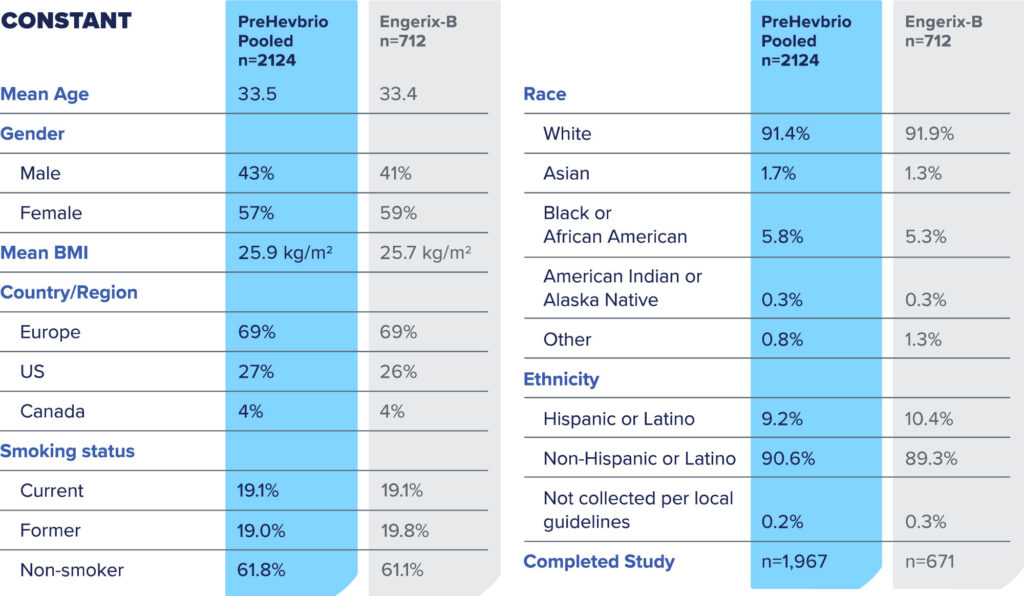
CONSTANT—Phase 3 clinical trial to evaluate lot-to-lot consistency1
CONSTANT Study Design
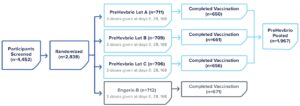
PreHevbrio was evaluated in a double-blind, randomized, four-arm, lot-to-lot consistency study of adults age 18 to 45 (n=2,838) across 35 sites in Europe, the U.S., and Canada.2
Seroprotection Rates in Adults Age 18-451,2
PreHevbrio achieved non-inferior SPR in adults age 18-45
Seroprotection Rates at Day 196
* Seroprotection was defined as anti-HBs titers ≥10 mIU/mL; SPR was defined as the percentage of participants attaining seroprotection.
Concentration of Anti-HBs Antibody Titers2
Antibody Titers at Day 196
* Seroprotection was defined as anti-HBs titers ≥10 mIU/mL; SPR was defined as the percentage of participants attaining seroprotection.
Indication & Important Safety Info
INDICATION
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
INDICATION
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PreHevbrio.
Immunocompromised persons, including those on immunosuppressant therapy, may have a diminished immune response to PreHevbrio.
PreHevbrio may not prevent hepatitis B infection, which has a long incubation period, in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common side effects (> 10%) in adults age 18-44, adults age 45-64, and adults age 65+ were pain and tenderness at the injection site, myalgia, fatigue, and headache.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women who received PreHevbrio during pregnancy. Women who receive PreHevbrio during pregnancy are encouraged to contact medinfo@vbivaccines.com or call 1-888-421-8808 (toll-free).
To report SUSPECTED ADVERSE REACTIONS, contact VBI Vaccines at 1-888-421-8808 (toll-free) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov
Please see Full Prescribing Information.